A research paper entitled Estimating the Financial Impact of Gene Therapy in the U.S. by The National Bureau of Economic Research (NBER) estimates that 1.09M Americans will receive gene therapy between January 2020 and December 2034. A relatively new science, many have heard of the technology but are unsure what it is or how it works. In this blog, we will look at the various types of therapies, their origins, similarities and differences, and how they are being used to treat a myriad of diseases.
What is gene therapy?
The roots of gene therapy (GT) date back to researchers at the National Institutes of Health. In 1990, a pediatric patient born with a rare genetic disorder known as severe combined immunodeficiency (SCAD) received the first gene therapy treatment. The new method was controversial at the time, but the therapy saved the child’s life after she stopped responding to other treatment options.
Gene therapy is the method of using genetic material to treat or prevent disease. There are two ways this procedure can be completed:
- In-vivo Treatment: Healthy gene copies are injected directly into the patient, either intravenously through an IV or locally to an affected organ.
- Ex-vivo Treatment: Affected cells are removed from the patient and genetically altered in a laboratory setting. The resulting healthy cell copies are then reinserted into the patient.
Gene therapy is most often used to treat disorders in the following therapeutic areas: oncology, cardiology, and blood disorders.
What is cellular therapy?
Cellular therapy (CT), also known as cell therapy, can be traced back to 1958 when a French oncologist used bone marrow grafts on nuclear technicians exposed to high radiation levels. He later went on to use a bone marrow transplant to treat a patient with leukemia.
Cellular therapy involves transplanting human cells into a patient to repair or replace their damaged cells. These cells can be living or genetically modified and are typically given to patients through blood transfusions. The cells can come from the patient receiving the treatment or from a donor.
Various types of cell therapies exist, and they can be used alone or in combination with other forms of treatment:
Dendritic Cell Therapy: In this type of cell therapy, new dendritic cells are grown in a laboratory from the patient’s blood cells.
Hematopoietic stem cells (HSC): The most common form of cell therapy, HSC is also known as a bone marrow transplant and is frequently used to treat blood cancers and other hematologic disorders
Stem Cell Transplant: Cell therapy is most often used to treat disorders in the following therapeutic areas: oncology, autoimmune and neurological disorders, and infectious diseases.
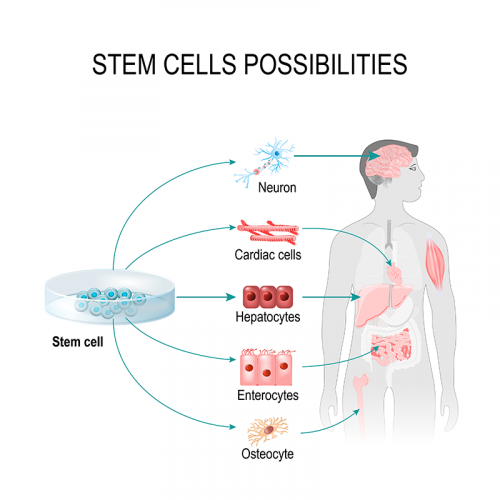
What is cell-based gene therapy?
Cell-based gene therapies use the technologies of both gene therapy and cellular therapy together.
- RNA Therapy: While most other therapy options focus on modifying the double-stranded DNA within a cell, RNA therapy focuses on the single strand of protein known as RNA. When the process focuses solely on the messenger RNA of the cell, the treatment is referred to as mRNA therapy.
- Epigenetic Therapy: This treatment focuses on disrupting the epigenetic changes within a cell. These changes are often called “tags” attached to the DNA. Researchers can turn the genes of specific genetic disorders on or off using these tags.
- Chimeric Antigen Receptor T-Cell Therapy (CAR-T): CAR-T therapy is a type of cell-based gene therapy. Once modified and implanted, the new chimeric antigen receptors attack the cancer cells directly.
CAR-T therapy is approved for use in patients who have been through another treatment option, like a stem cell transplant or chemotherapy, with no success. The pharmaceutical industry is very optimistic about the ability of this method to cure certain cancers.
Cell-based gene therapies are most often used to treat disorders in the following therapeutic areas: oncology, cardiology, and blood disorders.
What is gene editing?
Gene editing combines the science used in both gene and cell therapies to treat ill patients. Faulty elements of deoxyribonucleic acid (DNA) are removed from within the gene and corrected. This therapy can occur inside or outside the body using the patient or donor cells.
One type of gene editing is known as clustered regularly interspaced short palindromic repeats, or CRISPR. This tool is derived from the immunity properties found in bacteria and allows DNA to be cut at exact locations for targeted treatment within living cells. Because of its accuracy, CRISPR looks very promising in treating several genetic diseases. CRISPR therapy is currently being tested in various clinical trials, with the first FDA approval anticipated in 2023.
Are there currently any FDA-approved gene or cell therapy treatments?
In the United States, all new treatments must be vetted and approved by the U.S. Food and Drug Administration (FDA). This government entity oversees the clinical trial process throughout all phases as a new drug comes to market, approving and monitoring the individual protocols required for each study. The FDA must be advised of any protocol deviances or patient adverse effects and has the authority to put a trial on hold or end it immediately based on their study review.
Since 2017, there have been 27 gene and cell therapy treatments approved by the FDA for use in therapeutics areas such as oncology, and hematology, and several of note for use in rare diseases:
- Luxturna: In 2017, Spark Therapeutics won approval for the first virally-delivered gene therapy to treat rare genetic vision disorders known as inherited retinal dystrophy. Approved for use in both children and adults, Luxturna was shown to reduce vision loss by delivering an edited gene copy directly to the compromised cells in the retina.
- Zolgensma: In 2019, the FDA approved the gene therapy treatment Zolgensma for use in pediatric patients under the age of two with the rare neurological disorder spinal muscular atrophy (SMA). This one-time intravenous (IV) infusion treatment created by Novartis has been proven to significantly reduce the progressive effects of the debilitating genetic disease, which once meant a life expectancy of fewer than five years.
How can gene and cell therapy benefit rare disease patients?
Many rare disorders are genetic in nature, passed down from the genes of one parent, or resulting from a combination of inherited genes from both parents. The genes resulting in a rare disease can be dominant or recessive in a patient’s parents.
More than 7,000 rare diseases have been diagnosed, with over 80% having a genetic component. Sadly, three-quarters of these affect children; these disorders are often more severe, including chronic and progressive decline in pediatric patients. Traditional treatment methods have not always succeeded, so there is great hope for new and improved cell and gene therapy options. With scientific advances will come much-needed life-enhancing and life-saving treatments for many within the rare disease community.


